SGMO: Sangamo Getting Ahead Of Itself
Sangamo BioSciences has received a great deal of attention in recent weeks with the unveiling of a broad orphan drug therapy initiative at JPMorgan. This has overshadowed what appears to be weak data in its leading HIV drug candidate SB-728-T.
Background:
SB-728-T is an autologous zinc finger nuclease (ZFN) modified T-cell product designed to provide a reservoir CD4+ T-cells with genetically disrupted CCR5 receptors in HIV infected patients. CCR5 is used by the virus to bind and enter cells; individuals homozygous for the delta32 mutation in CCR5 are known to be highly resistant to HIV.
The clinical precedent for Sangamo’s work lies in the case study of an HIV infected patient (the “Berlin Patient”) who underwent stem cell transplant from a delta32 homozygous donor. Following the transplant, the patient saw a restoration of CD4+ T-cells and maintained viral loads below the limit of detection even with the discontinuation of all antiretroviral drugs.
The Data:
In what has been hailed as a potential leap forward in deriving an HIV “functional cure”, Sangamo offered intriguing Phase I data at ICAAC in 2011.
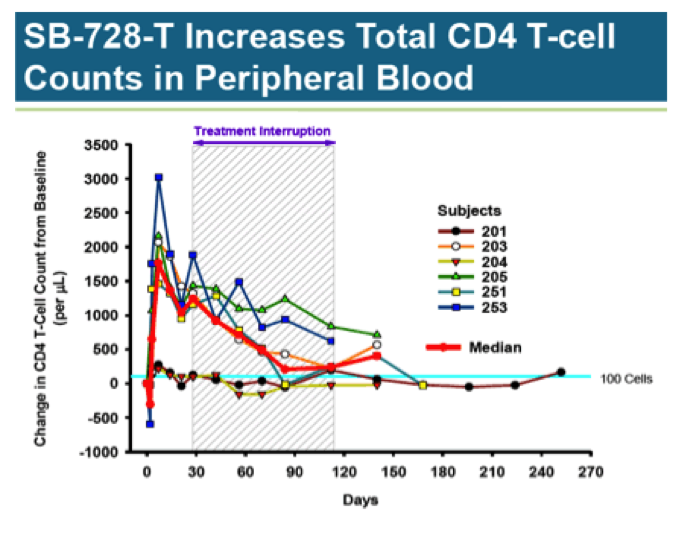
Of most interest is a 12-week HAART treatment interruption study involving 6 HIV infected patients with undetectable viral load (aviremic). Median CD4 T-cell counts for this group at baseline was 974/ul. Each subject received a single infusion of SB-728-T (10 billion cells); 4 weeks later, they were taken off all anti- retroviral drugs.
As you can see, CD4 T-Cell counts jumped quickly following treatment, more than doubling from baseline; however, it is uncertain whether the increase is due to real cell proliferation, or an artifact from activating the cells during preparation of the treatment. In any case, the number of CD4 T-Cells quickly peaks and begins a slow decline. Looking at subject 251, who showed a robust response, and 201, who had a minimal response, there appears to be a convergence at the 100-cell mark, indicating a sustained increase of only about 10% above baseline.
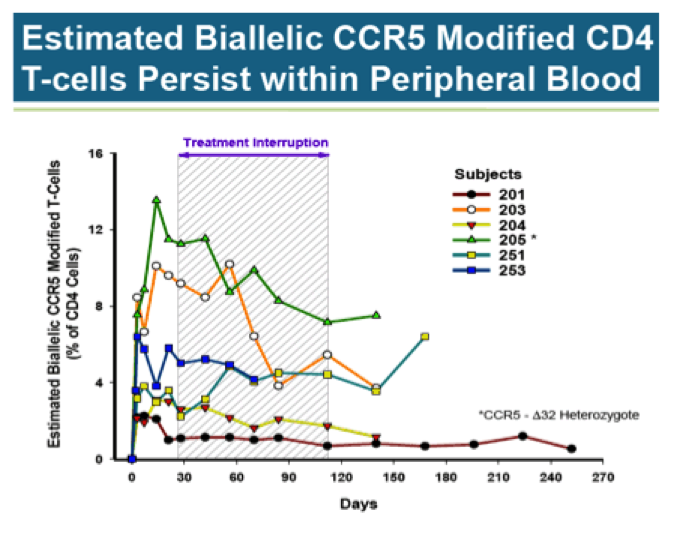
Here is another look at the data, only this time the focus is on percent of CD4 T-Cells with both copies of the CCR5 gene disrupted by ZFN (biallelic). The individual with the greatest biallelic modification by far is subject 205. It should be noted this subject is delta32 heterozygous (mutation at one CCR5 gene), gaining a natural advantage over other participants. Even so, biallelic CCR5 modified cells, on average, remain low as a percentage of all CD4 cells.
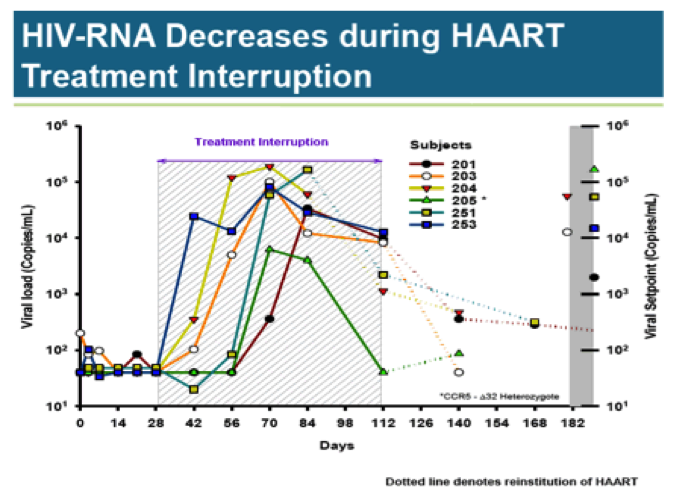
During the HAART treatment interruption, viral load rises rapidly in all subjects; only subject 205 is able to clear the virus. Two subjects required early reinstitution of HAART. The three remaining patients saw slight viral load reductions from peak levels.
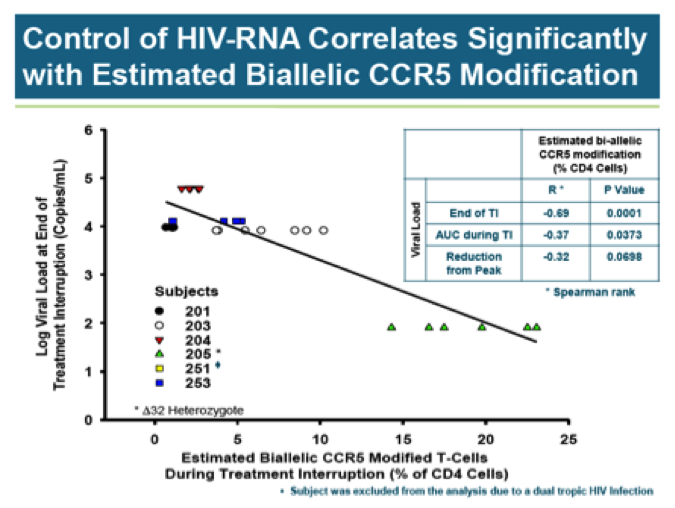
There does not appear to be any correlation between CCR5 modification and viral load. With the exception of the delta32 heterozygote, all subjects had similarly high viral loads prior to resumption of HAART therapy regardless of CCR5 modification levels. Of course, it is quite easy to draw a line between two points and this is what Sangamo has done in declaring a correlation HIV-RNA and biallelic CCR5 modification:
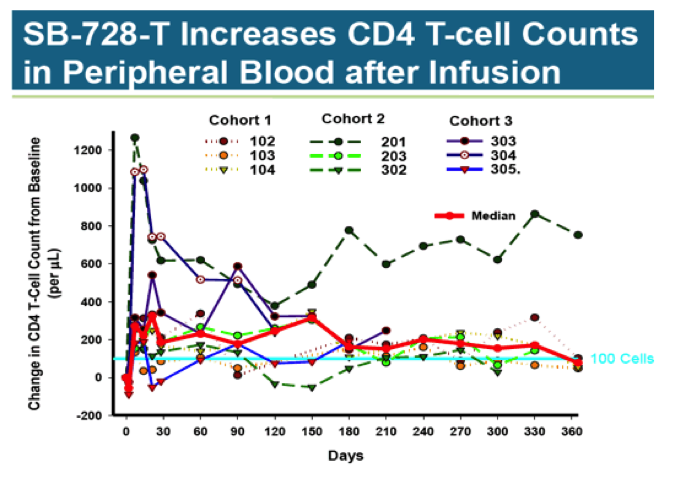
The second study looked at dose-escalation of SB-728-T in nine HIV positive patients on stable HAART. These patients, while aviremic, had low CD4 T-Cell counts of between 200 to 500 cells/ul. Three equal cohorts received single doses of 10 billion, 20 billion, and 30 billion cells.
As with the treatment discontinuation study, T-Cell counts rose substantially following infusion, only to drift down over time. At 360 days, the median increase was about 100 cells, a level that is unlikely to be clinically significant. There does not appear to be a dose-response relationship between number of cells dosed and change in T-Cell counts.
The studies are interesting but inconclusive. Sangamo has shown genetically altered cells reintroduced into the body can stay in circulation- albeit at low levels- for over a year. It was thought that exposure to HIV would select for and enhance levels of the modified T-Cells; this does not appear to be the case.
Because of what appears to be success in the single delta32 heterozygote, the company has added a cohort of delta32 heterozygous patients to the dose- escalation study. These patients will receive between 5 to 30 billion modified cells then undergo a 16-week anti-retroviral treatment interruption two months following the initial infusion.
Sangamo is also studying the use of cyclophosphamide (Cytoxan) to enhance T- Cell engraftment. The plan is to kill off much of the patient’s T-Cell population just prior to infusing the genetically altered cells in an effort to increase the proportion of biallelic cells.
“At least 9 HIV-infected subjects on HAART are being enrolled into 3 dose- escalating cohorts (3 subjects/cohort), and receive intravenous Cytoxan (200 mg, 500 mg or 1000 mg). Within each cohort, treatment is staggered so that each subsequent subject cannot be infused with Cytoxan until at least 2 weeks after the preceding subject. One day after receiving Cytoxan, subjects are infused with SB-728-T (5 to 30 billion cells). Six weeks after SB-728-T infusion, subjects with CD4 cell counts ≥500 cells/mm3 undergo a 16 week TI during which time their anti-retroviral therapy is discontinued. At the end of the TI, subjects with an undetectable viral load can remain off HAART until HIV RNA levels are detectable or their CD4 T-cell count drops below 500 cells/ mm3 for three consecutive weekly measurements.”
The two new studies have subtle but potentially important changes to the original treatment interruption protocol. First, the initial period following T-Cell infusion has been extended from 4-weeks to 6 and 8 weeks. Second, treatment interruption has been lengthened from 12 to 16-weeks. And finally, patients achieving undetectable viral load are allowed to remain off HAART, providing the first sense of duration of response.
Preliminary data will be presented in 1H, with final results by the end of year. If results are good, Sangamo hopes to partner the program. While there are some who believe the company can move into a Phase III based on expected results, such views are wildly optimistic. The trials may be called Phase II, but they are truly exploratory studies; much work remains before a pivotal trial can be initiated.
New Projects:
Even as SB-728-T remains the lead program, management has shifted much of the investor community’s focus on a slew of pre-clinical and discovery stage projects. Gene therapy is back in vogue and zinc fingers are novel and sexy. Sangamo has upped the ante from HIV “functional cures” to “genetic cures” for diseases ranging from hemophilia to Huntington’s disease to essentially any monogenic disease.
INDs in hemophilia A and B are expected to be filed in 2014 along with one for the treatment of HIV with modified stem cells rather than mature T-Cells. An IND for Huntington’s disease in partnership with Shire is expected in 2015. Filings in two lysosomal storage diseases are also expected that year.
The Sangamo zinc finger approach is now emphasized as a “platform technology”. The possibilities are indeed exciting and easily capture the imagination. It is easy to forget the difficulties new medical technologies face in paving the road to success. Investors would be wise placing minimal value on these pre-clinical projects until human proof of concept has been established.
Summary:
Results from Sangamo’s lead HIV program thus far has been underwhelming. Tangential studies looking at delta32 heterozygotes have some scientific underpinning, but clinical observations are based on a single observation. Even if ultimately successful, this method will be limited to a very small minority of patients with the particular mutation.
The Cytoxan study designed to increase the proportion of biallelic cells is also based mainly on data from the heterozygote. That subject had the highest level of biallelic T-Cells as a percent of all T-Cells, and saw a complete reduction in viral load. With both studies dependent on observations of a single individual, there are significant risks to the trials. Another concern with the Cytoxan study is toxicity as the chemo dose is escalated.
Sangamo is an early stage company with a promising technology. It still has a long way to go to prove itself. Valuation looks to be getting rather heady. Proceed with caution.
Disclosure: Author has no position














Thanks for this very helpful report. Yes – SGMO may be reading too much into these very early stage results. With the recent news of the newborn “cured” of HIV, everyone seems to be saying they too are stepping up development of promising candidates. The attention is great and maybe that will result in increased research funding after a string of disappointments. There are many interesting vaccine technologies being developed and tested from university level to the Thai trials to upstarts like GeoVax. All seem to raise more questions than answers which calls for potential investors as well as the vaccine researchers to proceed with caution.
Totally massaged data from HIV, not even clinically meaningful. I am short so I am biased.
Agree just one subj with undetectable viral load doesn’t make a trend. I’ve seen the correlation plot a few months ago and obviously, removing sbj 205 leaves with a poor correlation. However, their attempts to increase engraftment leading to higher biallelic modification can still pay off. The design of Ph2 allows for a better assessment of impact on VL relative to Ph1 (HAART will not be re-instated if VL is dropping). Odds of undetectable VL across all sbjs is still low. The Ph2 studies are not blinded/controlled, they must have some idea of how sbjs are responding.
I have considered buying puts, but think the r/r may be limited due to the company shifting investor focus to monogenic diseases. A failure in HIV may not drop share prices too severely.
tried buying $SGMO puts before. A lot of long term $$s led this rally, there cud be a lot more. ZFN is way too cool.