Trillium Therapeutics Inc. (TRIL) – Nasdaq
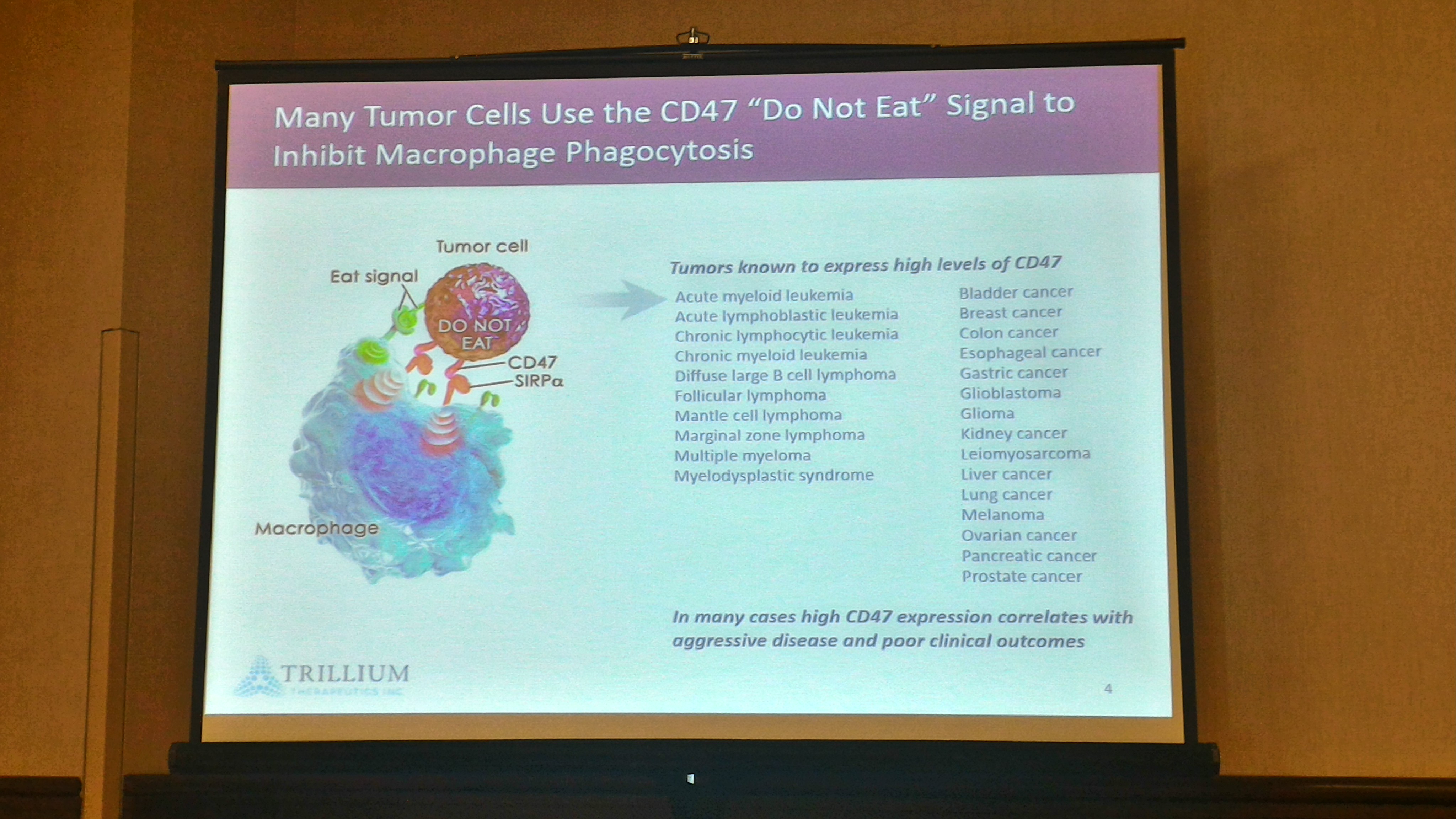
Trillium Therapeutics (TRIL) presented at BloomBurton-2016 Healthcare Investor Conference in Toronto on May 3rd, the same as last year the speaker was TRIL’s Chief Scientific Officer (CSO) Dr. Bob Uger, he took the stand in Hall A (the largest room at the conference) that was full with investors, press and even a TV crew, showing the high interest around Trillium in Canada as well. He was very informative during the presentation, focusing on the CD47 development, mentioning that any tumor cells use the CD47 “Do Not Eat” signal to inhibit macrophage phagocytosis.
The CD47 program holds great promise as both monotherapy and combination therapy with other anti-cancer agents, as TRIL are looking for a combination therapy with other immunological agents such as approved cancer antibodies, T cell checkpoint inhibitors (e.g., anti-PD-1) , Cancer vaccines, Oncolytic viruses or CAR T cells, TRIL already evaluating SIRPαFc in preclinical combination studies to find out the best agent to advance to clinic. TRIL has broad clinical potential in both hematological and solid tumors.
After the presentation, I had a meeting with Bob, and had the opportunity to ask him several questions, started with the first question in mind, are you looking after a particular anti-PD-1 for a combination therapy:
He replied that they are looking for a several combination, looking for combos with many companies and there are so many interesting options in the market, TRIL’s CD47 blockade can enhance the potency of anti-cancer antibodies and promote T cell responses, and would be great to see the enhancement to an approved drug. But they will inform the investor as soon as the decision is taken.
Q: Can you give us an update how the Phase 1 trial is going.
The open-label, Phase 1a/1b trial of TTI-621 in patients with relapsed or refractory hematologic malignancies is running well, no timeline to share right now, it depends on what dose we ends up at, till we hit the MTD (Maximum Tolerated Dose), we are hoping to have updates at ASH-2016 this year, that is our plan to have enough data to present, we are very optimistic to have a meaningful data set to present at that date.
The trial supposed to enroll around 156 patients, we have 5 trial centers and the last tow centers already started enrolling patients as well, after we hit the MTD, we will look for the expansion phase on patients with a variety of hematologic malignancies that will be treated at the optimal dose to further define safety and to characterize efficacy, it will be 12-15 patients per cohort.
Q: What will TRIL present at ASCO-2016?
At ASCO we will present “Trial in Progress category” it’s not data, but explaining what the trial is, it will be a poster presentation, entitled “ SIRPαFc, a CD47-Blocking Cancer Immunotherapeutic, Triggers Phagocytosis of Lymphoma Cells by Both Classically (M1) and Alternatively (M2) Activated Macrophages ”.
Q: Do you think that CD47 can work as a monotherapy or only as a combination with other agents?
I think it can work as monotherapy as well, in general people feel that every thing is moving to a combination direction, we will start with a monotherapy to see the therapy activity, we are optimistic from our preclinical data, we are thinking of combination as well, and trying to advance it to the clinic, we like the anti-PD1 that mechanistically make sense and obvious combination, other things just like anticancer antibody, combination like Rituxn, and some prospects to chemotherapy combination as well, we will decide what is the best combination for us and then advance it to clinic. For my question of when you see a combo study in clinic, he replied we are working hard to make it happened this year 2016, it’s difficult to say right now, we have to see the data and then we will decide.
Q: What about partnership, any pharma companies interested after TRIL started the Ph1 clinical trial?
Well are still in early stages of our program. We keep our options open, have a lot of interest, but we are excited of what we have in our hands, keep things open till we get what we think we deserve, we are looking after our investors best interest. We will choose carefully the partner that will bring us and our investor a real added value and help us to step up for the next stage.
Q: Do you think the human data that will be presented by Stanford at ASCO will affect TRIL as well?
We will see what they show, I have no idea what they will present, I think any success by anyone else in the field will have a positive impact, but it’s important to keep in mind that we are taking a different approach, we are not going after the same target, and there is an important difference between both programs, we still have the potential to be best in class.
Q: What do you think the next meaningful catalyst for TRIL?
I think the early clinical data at ASH-2016 is the next significant infliction point for us, specifically investors want to see what is going on with the anemia and the differentiation between us and other programs, it will be our milestone, we hope to show that we have no anemia as SIRPαFc has much lower binding to human RBCs compared to anti-CD47 mAbs, with the potential to be best in class through lower hemotoxicity (anemia) and more favorable PK (no RBC “antigen sink”) and non-interference with blood typing.
Next is the expansion phase where we will choose the next target like AML, Myelodysplastic syndrome, Chronic lymphocytic leukemia, Hodgkin lymphoma, Indolent B cell lymphoma, Aggressive B cell lymphoma, T-cell lymphoma, Multiple myeloma.
Q: What about the new acquisition Flourinov and when we expecting an IND work?
We just started, we have no specific guidance yet, we just recently acquired the asset, they have a priclinical data but they hadn’t enter into IND enabling phase, need to do the scale up, and some other work to advance the program. This acquisition is a great expansion opportunity for us at a very favorable terms.
Disclosure: Author is long TRIL.