The Adcom is over and Eteplirsen was voted down. A final decision still remains for approval. Much was discussed in the Adcom, the key arguments were over the following:
1. Adequacy of dystrophin creation.
2. Efficacy of 201/202 Trial vs Historical Controls
Both are intertwined.
Adequacy of dystrophin creation:
FDA had little faith in early dystrophin measurements due to the single lab processing of tissue samples and perceived sloppiness of assays conducted.
After much back and forth, a new agreed upon method was deemed satisfactory to FDA for detecting the protein and used in the 180 week biopsies.
The question was then whether the degree of dystrophin increase as detected by western blot and immunohistochemistry were sufficient to have a physiological effect on DMD boys.
With the DMD science community still debating this subject, Sarepta had no answer.
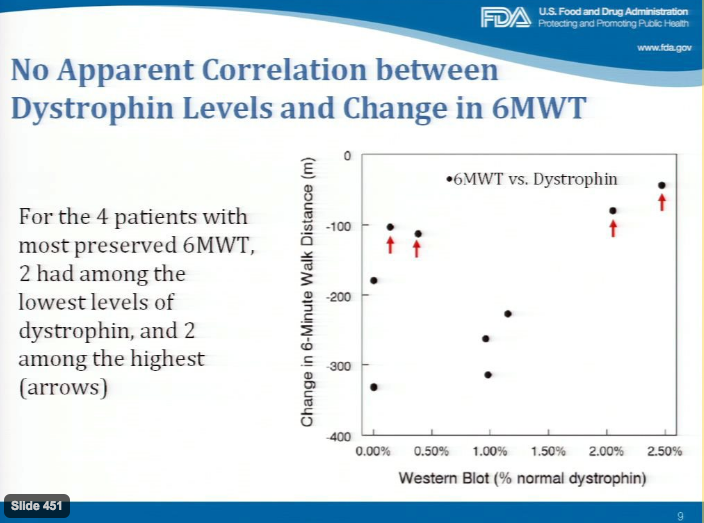
In this graph, FDA pointed to a lack of correlation between dystrophin expression and change in 6MWT score (Adcom):
In the above, less change in the 6-Minute Walk Distance is better; those with red arrows have the best performance. While no threshold dystrophin level is currently known as a requirement for improvement in DMD, this graph calls into question a lack of randomness in patient selection. The two high performers with lower dystrophin may simply be healthier than others.
Missed in this argument is eteplirsen can and does lead to significant de novo dystrophin production to a level overlapping with that found in Becker’s. This is a major find in and of itself- no other drug has produced functional dystrophin in humans. Perhaps not all boys achieve the same level of benefit, but some certainly do. Repeatedly saying eteplirsen only increases dystrophin to 0.9% of normal is simply wrong.
Efficacy of 201/202 Trial vs Historical Controls:
This issue is a major point of contention. Going back a step, the 201/202 trial is a prospective, uncontrolled, open label study, making it prone to errors, particularly through bias. Historical controls may be misleading due to differences in patient population, treatment, or other factors. Comparison to multiple historical controls help alleviate some of this. A matched historical control, under these circumstances, is the best possible option.
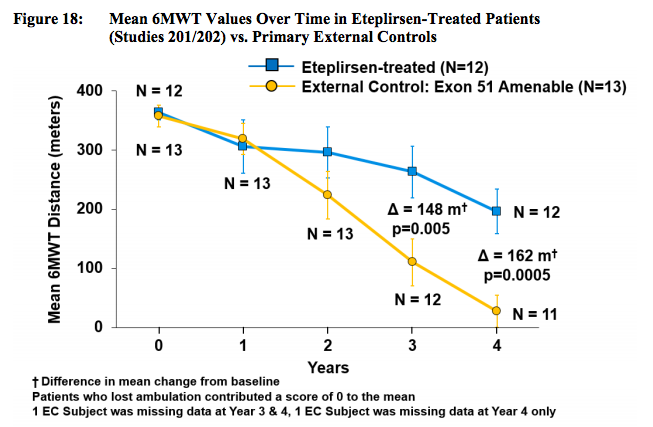
As we know, eteplirsen treated patients demonstrated a slower decline in the 6MWT compared to a multitude of longitudinal DMD studies, this is suggestive of activity although questions remain. FDA has stated Sarepta’s open label trial has made statistical analysis impossible. Be that as it may, a graphical depiction of Eteplirsen vs. matched external control makes view an improvement large enough to be clinically significant. (SRPT Docs):
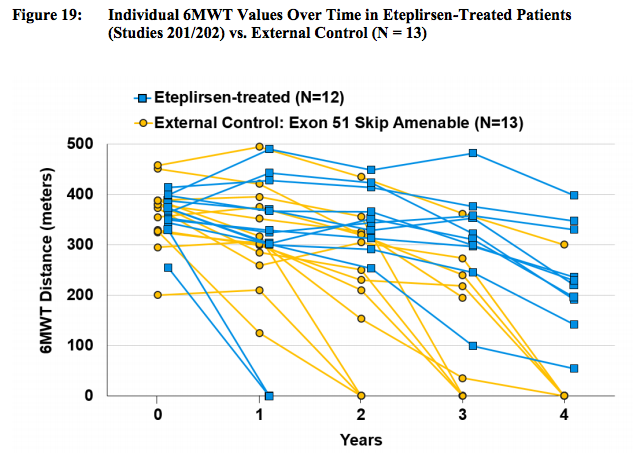
Patient level data gives a more detailed look. (SRPT Docs):
These graphs appear to show a clear difference in 6MWD trajectory for eteplirsen treated patients relative to control.
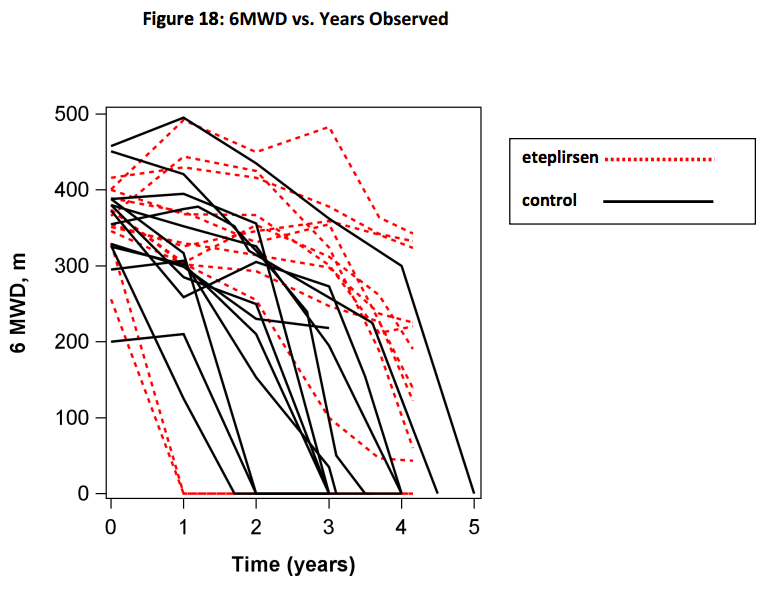
Not surprisingly, FDA saw things differently (FDA Docs):
The agency proposed that rates of decline are similar between the groups in this chart, with substantial overlap between the groups. On first glance the graph appears to support FDA’s assertion. But the curves are clearly divergent with only a single patient from the control cohort roaming into eteplirsen territory. (Note: The FDA chart seems to deviate significantly from the one provided by Sarepta leading to a poorer outlook.)
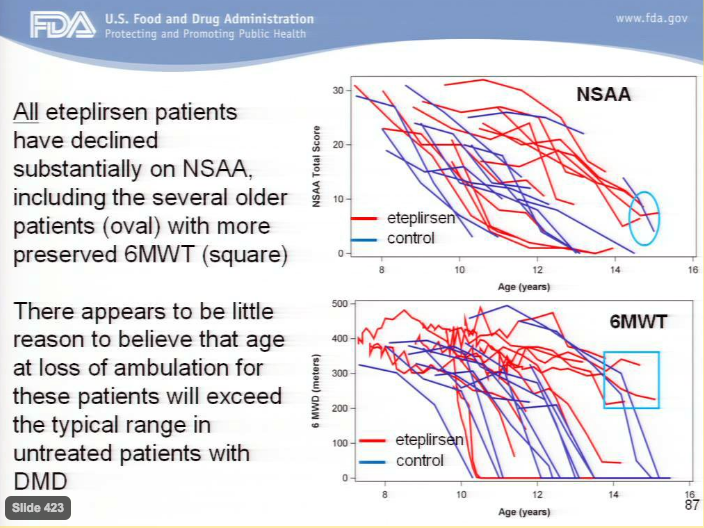
Unlike the sponsor, FDA signaled a preference for a 6MWT to age comparison rather than time from treatment onset. Below shows correlations between NSAA score and 6MWD to age (Adcom):
Rather than focusing on the clear group of eteplirsen treated patients who outperform all others, FDA focuses on one or two control patients with relatively high scores at their age. Missing the forest for the trees, FDA fails to see an entire forward shift in the Eteplirsen treated population.
Picking at the FDA data, it is notable the two controls (blue lines) with longest time before loss of ambulation had the two highest 6MWD scores, indicating a steeper decline than eteplirsen.
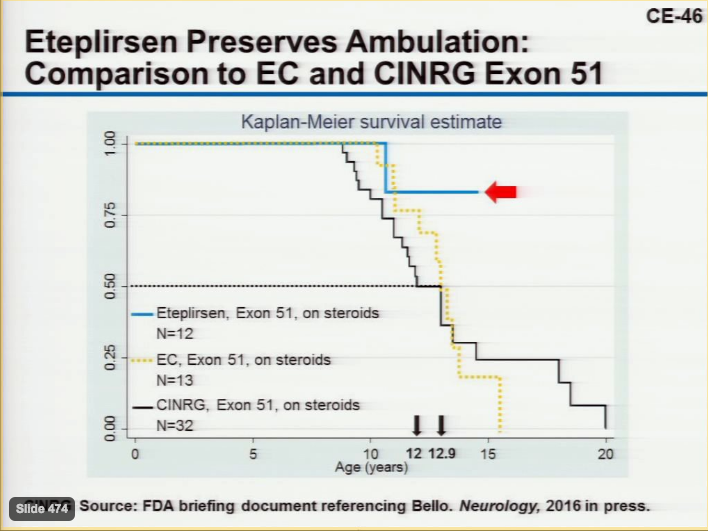
One last item, a Kaplan Meier curve from a subset of subjects in the CINRG study was presented to demonstrate ambulation in a substantial number of DMD boys can be retained until age 16 and beyond (Adcom):
This slide was altered by Dr. Mendall to include eteplirsen and external matched control boys along with a simple statistic: median time to Loss of Ambulation (LOA). Median time to LOA is 12 for CINRG, 12.9 for External Controls (EC), and not yet reached for Eteplirsen. FDA has suggested some Eteplirsen boys may be close to losing ambulation in the near future based on NSAA scores. Even under this assumption the overall picture is unchanged; eteplirsen leads to a beneficial shift in time to Loss of Ambulation.
Summary:
Eteplirsen treatment leads to de novo dystrophin production, reaching 2% to 2.5% of normal in some patients, levels found in milder BMD disease. This novel dystrophin has been confirmed by western blot, immunohistochemistry, and RT-PCR.
The sample size is small, but durability of effect and large divergence in performance of functional tests from external controls is convincing that superiority is not due entirely to chance or other factors.
There is reasonable evidence to conclude Eteplirsen is an efficacious drug.
Disclosure: Author is Long Sarepta