MDNA.TO (TSX – Canada), MDNAF (OTCQB – USA)
Medicenna is a clinical-stage immunotherapy company developing novel, selective IL-2, IL-4, and IL-13 Superkine molecules exclusively in-licensed from Stanford University. The Superkines are engineered versions of naturally circulating immune system cytokines, designed to enhance cancer-killing properties of immune cells while enabling systemic therapeutic dosing by minimizing their side effect risks at high exposure levels. Medicenna’s lead immuno-oncology asset in a Phase 2 clinical trial is MDNA11, an enhanced Interleukin-2 (IL-2) Superkine being studied in melanoma and other solid tumors in a monotherapy setting and also in combination with Keytrudaâ under a collaboration agreement with Merck.
MDNA11 will be the main driver for the company valuation both in terms of near-term data catalysts and value creation expected in mid-2024 with cash runway extending into Q2 2025. But the company also has an interesting pipeline of Superkines that create additional upside opportunity. These include a superior alternate to Antibody Drug Conjugates (ADCs) using Medicenna’s proprietary Empowered-Superkine platform, a Phase 3 ready candidate, bizaxofusp (MDNA55) with compelling data in glioblastoma (GBM) where there is huge unmet need.
At the pre-clinical stage Medicenna has an exciting BiSKIT (Bifunctional SuperKine Immuno Therapies) platform for treating immunologically cold tumors with a bispecific approach using IL-13 superkines for targeting and masking.
Finally, in early discovery are IL-2 Super-antagonists (MDNA209) and IL-4 Super-agonists that have demonstrated potential in various autoimmune and neuro-muscular diseases, expanding Medicenna’s Superkine platform beyond oncology. In the near term, we believe MDNA11, as a potentially best-in-class IL-2, with positive news flow in the monotherapy and combination settings could drive the stock price significantly higher.
MDNA11 (IL-2 Superkine) Immunotherapy
IL-2 has a long history in the world of immunotherapy and was actually the first ever Immuno Oncology (IO) treatment approved by the FDA for renal cell carcinoma (RCC) and for metastatic melanoma over 25 years ago, in recombinant form and under the brand name Proleukin. The anti-tumor activity of IL-2 was thus well-established, proving to cure around 10-15% of RCC and melanoma patients and representing a major leap forward at that time. Despite promising efficacy, Proleukin carried serious, life-threatening side effect risks (and could only be administered in the ICU) because of its promiscuous binding to non-immune cells and involvement in multiple biochemical pathways. Since recombinant IL-2 has a short half-life, Proleukin requires a heavily burdensome dosing regimen of 3 infusions per day for 5 days. With much of the intricacies of IL-2’s biochemical activity uncovered in the years since, many companies are investing in new approaches to capitalize on its favorable qualities and minimize its downsides. Medicenna is at the forefront of these efforts with what we believe is the most promising IL-2 optimization effort.
MDNA11 was engineered as a superagonist IL-2 through several rational optimizations. Since IL-2 has multiple functions, combating tumors via activating cytotoxic T cells and Natural Killer (NK) cells but also dampening the immune response through Regulatory T cells (Tregs) via binding to the IL-2 alpha receptor (CD25), its efficacy was somewhat limited. Moreover, the IL-2 receptor alpha subunit is also expressed on endothelial cells and responsible for severe toxicity including eosinophilia and life-threatening vascular leak syndrome. Through site-specific mutations using Nobel-Prize winning directed evolution techniques, Stanford scientists enhanced the efficacy of IL-2 with stronger binding to the IL-2 receptor beta/gamma subunits (IL-2Rβ𝜸), as this was known to stimulate cytotoxic T cell and NK cell proliferation underlying its anti-tumor activity. At the same time, IL-2 alpha receptor binding was eliminated from MDNA11 through specific mutations, thus removing the Treg limitation on efficacy as well as its major avenue for toxicity. The short half-life of IL-2 (0.2 to 1.4 hours after IV infusion) not only necessitated burdensome dosing every 8 hours for 5 continuous days, but also pushed the dose of Proleukin very high to achieve anti-tumor efficacy, which also drove up the concomitant IL-2 alpha binding and magnified the toxicity. The third key element to MDNA11’s optimized structure is its fusion to albumin via a (G4S)3 linker peptide (commonly used to create fusion proteins). The linker and fusion with albumin avoid spatial interference with the important beta/gamma binding regions of IL-2 within MDNA11. Because albumin has a much longer half-life, MDNA11 can remain circulating in the bloodstream much longer than standard IL-2. This enables MDNA11 to be dosed conveniently once every 2 weeks while achieving the pharmacodynamic effect required for consistent IL-2 based T lymphocyte and NK cell activation and without the need for a massive dose that would risk side effects like Proleukin. Coupled to the removal of IL-2 receptor alpha binding, this results in prolonged anti-tumor immune system activation at reasonable and infrequent doses without triggering IL-2 toxicities. Intriguingly, albumin helps MDNA11 accumulate at the site of the tumor and draining lymph nodes for additional efficacy boost. While many companies are investing in ways to unlock IL-2 with next-gen approaches, Medicenna appears to have the “better mousetrap” that solved this puzzle like no other. (See below for limitations of attempts by other companies at an IL-2 solution).
Current MDNA11 data including recent update from the Phase 1/2 ABILITY Trial at SITC 2023
In August, Medicenna presented data from the ABILITY Trial, a Phase 1/2 trial testing MDNA11 in multiple heavily pretreated solid tumors. That early data showed tremendous promise and validated the precise engineering of MDNA11 by demonstrating a highly tolerable safety profile that was unique in the world of IL-2. Across 20 patients, no grade 4 or 5 AE’s were observed, and the vast majority of AEs were grade 1 or 2. No dose limiting toxicities were observed and the maximum tolerated dose was not reached. Most importantly, no eosinophilia was observed (a known precursor to vascular leak syndrome caused by IL-2 toxicity). Pharmacodynamic data also validated MDNA11’s engineering since, consistent with the IL-2 mechanism of action, it showed a continual lymphocyte elevation in treated patients, and impressively it achieved this across most of the duration of its 2-week dosing intervals. The albumin linkage has quite clearly dramatically transformed IL-2 from its native half-life of 0.2-1.4 hour. Additional data focusing on the targeted, higher therapeutic dose levels showed important ratios of CD8+ lymphocytes to Tregs with fold-increases in this ratio upon treatment consistent with IL-2 efficacy and selectivity for the IL-2Rβ𝜸 as intended. The increase in CD8+/Treg ratio to approximately 50 due to MDNA11 is more than 5-fold elevated over baseline and is even higher than those achieved by Proleukin, on a cross-trial basis. Although the focus of the early dose escalation data was on safety and pharmacodynamics, promising hints of efficacy were seen among a small number of patients who received higher doses. Medicenna reported a confirmed partial response (PR) in a metastatic pancreatic cancer patient and a very long duration stable disease (SD) of greater than 18 months in a metastatic melanoma patient who was treated with two prior lines of IO. A competing IL-2 drug program (from Mural Oncology, see below) has failed to show monotherapy PRs in dose escalation, so this activity already being seen with MDNA11 is extremely promising.
Medicenna learned important lessons from the early dose escalation data. The 90μg/kg dose level showed apparent optimal immune cell activation (importantly, without any meaningful associated eosinophil increase) and was chosen as the go-forward dose. As they move into the dose expansion portion of the trial, where more patients will receive the targeted therapeutic dose level, the company is adjusting the trial criteria to match up more favorably with those patients who experienced good results on MDNA11. Since early efficacy was seen in patients with tumors having microsatellite instability (MSI high) and mismatch repair deficient (dMMR) genetic signatures as well as prior response to checkpoint inhibitors (but subsequently progressed), additional patients added to the dose expansion will be selected for these characteristics and will also have fewer lines of prior treatment. Earlier treated patients like this will also be more likely to have baseline lymphocyte counts of >1,000. These criteria, all known drivers of efficacy for active agents with the IO mechanism of action, should naturally lead to a higher percentage of responses in subsequent patients added to the trial. The company’s latest update at SITC in November shows this already panning out as hypothesized.
At SITC 2023 held in early November, the latest updated data for the dose escalation showed another monotherapy PR in a melanoma patient who had earlier progressed on checkpoint inhibitor therapy. This patient received the 90μg/kg dose on a faster dose escalation schedule and impressively showed 70% tumor shrinkage by the first scan (at 10 weeks). Additional durable SD’s (>6 months) were also observed. Overall, among 22 patients reported at SITC, 15 patients received the higher doses of MDNA11 (60, 90 or 120 mg/kg) and only 9 patients would have met the inclusion/exclusion criteria for the Phase 2 monotherapy dose expansion. Impresssively, 3 of the 9 patients (33%) had durable stable disease (lasting 6 to 18 months) and 2 of 9 patients (22%) had a partial response for a disease control rate of 55%. This indicates that the patient population selected for the monotherapy dose expansion at 90μg/kg will likely have better outcomes with future updates continuing to show dramatic monotherapy response rates differentiating MDNA11 from the modified IL-2 field.
Upcoming readouts and catalysts for MDNA11
On October 25th, Medicenna successfully dosed the first patient in the dose expansion cohort (n=40 anticipated enrollment) of the Phase 2 ABILITY trial and announced commencement of the Keytruda + MDNA11 combination cohort on January 9, 2024. Full data from the monotherapy dose expansion study will be presented in the first half of 2024, plus initial data from the Keytruda combo around the same timeline. While full dose escalation cohort data will be available at a scientific conference, AACR and/or ASCO in the first half of 2024, we believe an incremental trickle of data updates may precede the full readout. Based on the timing of the first scan of the melanoma patient with PR reported at SITC and follow-up scans of pancreatic cancer patient who continued treatment after 100% reduction in baseline target and non-target lesions (not classified as a complete responder according to RECIST 1.1 criteria due to development of a new lesion during a six week vacation when patient was off-treatment), we expect additional updated scans on these and other patients during the Q1/2024 timeframe. With the first patients expected to be dosed imminently in the Keytruda combination trial, first scans could take us to the March/April timeframe, implying that we will likely have data updates, for both the monotherapy dose expansion and Keytrudaâ combination escalation arms, in Q2/2024. We expect these potential data updates to serve as major catalysts and inflection points in the stock since the monotherapy response rates will likely be enhanced by careful patient selection, and this will establish MDNA11 as the superior IL-2 approach after already showing signs of stark differentiation in its dose escalation data.
Competing IL-2 Projects Riddled with Failure
There have been multiple failed attempts by biotech and large pharmaceutical companies at enhancing the IL-2 efficacy and safety profile, but we believe these failed because they lacked one or more elements of the comprehensive approach of MDNA11. Nektar Therapeutics’ Bempegaldesleukin (Bempeg) was the highest profile failure, which followed a massive $1.85 billion upfront investment from Bristol Myers Squibb after early clinical trial data, although the program had no signs of monotherapy activity. Nektar sought to increase the half-life of IL-2 (Proleukin) via pegylation and combine it with PD-1 inhibitor Opdivo to enhance the efficacy of checkpoint inhibition. However, half-life extension through pegylation does not rebalance the NK cell and CD8+/regulatory T cell equation, so it fails to address one of the key limitations to IL-2’s efficacy. Furthermore, the longer a standard IL-2 cytokine circulates, the more chance it has to induce toxicity, which is what bempeg simply achieved in its Phase 3 trial with BMS resulting in failed program. Medicenna has addressed both of these issues with selective enhancement of beta-gamma subunit binding for superagonism to rebalance the T cell equation, coupled to the elimination of alpha binding to dampen toxicity. MDNA11’s bound albumin fusion recapitulates the goal of pegylation but with a simpler manufacturing process and supported by albumin’s ability to accumulate in the tumor and tumor draining lymph nodes (location where immune cells are trained to attack cancer). Addressing only IL-2’s half-life as Nektar did is simply not going to cut it.
Sanofi also paid $2.5 billion to buy Synthorx, primarily for its lead asset THOR-707, which was another attempt at IL-2 optimization. With THOR-707, Synthorx attempted to use site-specific pegylation at a novel amino acid to prolong half-life and prevent alpha receptor binding at the same time (because of the inability of the replaced amino acid to bind properly). This attempt also went up in smoke, but notably while THOR-707 improved on IL-2’s half-life and cut down on alpha binding, it lost some of its affinity for the IL-2 beta-gamma receptor and missed the third element captured by MDNA11. Engineered superagonism at the beta-gamma receptor within MDNA11 has already demonstrated a remarkable shift in the balance of lymphocyte stimulation to CD8+ T cells and NK cells beyond the efficacy reached by Proleukin.
Roche made another attempt at IL-2 optimization with an antibody-drug conjugate, but unsurprisingly this failed to sequester IL-2 within the tumor microenvironment sufficiently, and like all other ADCs, released a significant amount of payload (IL-2) into the systemic circulation. This resulted in dose-limiting side effects and failed to escape the toxicity problems of IL-2.
Additional variations on these themes are ongoing among a number of companies. One other approach we will highlight is that of Mural Oncology (MURA), since it is somewhat further along in development. Mural was recently spun out from Alkermes (ALKS) and brought further attention to the modified IL-2 space among investors. Mural’s modified IL-2, nemvaleukin, is a different type of fusion protein, this time including a high affinity IL-2 alpha receptor chain attached to the IL-2 molecule in a stable fusion to permanently block it from binding the IL-2 alpha receptor while also improving IL-2’s half-life. However, nemvaleukin’s IL-2 half-life improvement pales in comparison to Medicenna’s albumin fusion, and this necessitates daily infusions for 5 consecutive days of a 21-day cycle. This creates multiple problems. On one hand, this dosing regimen remains inconvenient and far inferior to a Q2W dosing with MDNA11. Yet it also puts a cap on nemvaleukin’s potential efficacy since a 5-day dosing period cannot extend the lymphocyte elevation continually much beyond those initial 5 days until the next dose of checkpoint inhibitor in the prolonged 21-day cycle. Nemvaleukin also fails to enhance beta/gamma receptor binding and sacrifices some of the affinity of native IL-2 due to conformational change with the alpha chain fusion attached. We believe these factors may be behind Mural’s limited efficacy demonstrated so far. Notably, whereas MDNA11 has now shown multiple PRs and durable SD’s in dose escalation, nemvaleukin failed to demonstrate any PRs during the dose escalation phase (with only transient SD’s), and has only since demonstrated PRs in the dose expansion phase with its latest data update (at the rate of a few PRs per 75 patients). At the same time, this follows the expected pattern of increasing efficacy in the dose expansion part of the trial, which is only more bullish for subsequent MDNA11 data, as we noted above. The toxicity of nemvaleukin was also surprisingly high based on its theoretical avoidance of alpha receptor subunit binding. The trial data showed 76% Grade 3/4 events with nemvaleukin. This would seem to suggest the alpha chain fusion’s inhibition of alpha receptor binding is incomplete, or that the fused alpha chain itself contributes to tolerability issues not observed with MDNA11 (or both). We maintain a healthy skepticism around nemvaleukin and expect it to continue to show inferior results to MDNA11.
Werewolf Therapeutics (HOWL) is another current competitor in the modified IL-2 space, but they are trailing behind MDNA11 in the development path. Werewolf’s WTX-124 is an attempt at enriching the IL-2 (identical to Proleukin) at the tumor microenvironment while shielding it from access to cells in the systemic periphery. Although the safety profile thus far (only grade 1/2 AE’s at very high doses) appears to validate the shielding and tumor microenvironment localization aspects of the design, we note that native IL-2 even when sequestered in the tumor microenvironment would still run into the problem of activating Tregs. This would likely limit the efficacy by counterbalancing anti-tumor activity with immunosuppressive regulatory T cell activation, either preventing responses or limiting response durability (or both). This seems to be playing out so far in Werewolf’s dose escalation data. So far, the company has provided high doses (12mg) of their shielded molecule exclusively in IO-sensitive tumors and has only observed 2 “unconfirmed PRs.”. As they now move to 18mg dosing, this represents a 3-fold higher amount of drug compared to the 90μg/kg (6.3 mg for 70 kg body weight) dose of MDNA11 selected by Medicenna. What is striking is that even though WTX124 is masked, they are seeing eosinophilia, a peripheral phenomenon which is a precursor to vascular leak syndrome, a side-effect consistent to what has been seen in the past with Proleukin due to alpha binding. To date, eosinophilia has not been an issue with MDNA11. Overall, it seems that WTX is being activated in peripheral circulation and/or the unmasked IL-2 is leaking out of the tumor microenvironment (TME) due to its small size after protease activation. Also, there is no way of knowing if the entire systemic dose is actually being unshielded and activated by proteases in the TME. What is also conspicuously missing in their SITC presentation is the absence of any data on Treg expansion in the TME or for that matter, relative changes in the population of CD8, NK and Treg populations in systemic circulation. These important data would have provided confirmation of WTX-124’s ability to retain activity in the TME and not in peripheral circulation.
Xilio (XLO) is another competitor with an approach similar to Werewolf’s relying on proteases in the TME to unshield XTX202 and its subsequent activation. However, unlike WTX124, they have engineered out the alpha binding activity and only retained the intermediate affinity IL-2-beta-gamma binding without any effort to enhance its beta-gamma activity. They remain in dose escalation studies with XTX202, and so far, have not observed any PRs but did achieve some SDs at higher dose levels (up to 4 mg/kg), which bears watching as they dose escalate further. XTX202 requires much higher dosing because of the masking domain and requirement to get enough drug localized at the TME for activity. Although the phase 1/2 data showed increases in CD8+ T cells and NK cells at higher doses tested, a peculiar side effect of lymphopenia was also observed in ~10-15% of patients, consistent with signs of peripheral activity. A decrease in lymphocyte counts is certainly a surprising AE for a masked IL-2 molecule, so this will also bear watching going forward. Overall, XTX202 is demonstrating decent safety at much higher doses than MDNA11 or WTX214 but seems to lack meaningful clinical activity so far. This may be attributed to poor activation of effector cells due to inferior binding of the “not-alpha” IL-2, which is not the case with MDNA11 which has a 30-200-fold increased affinity to the beta-chain when compared to IL-2 (i.e “not-alpha” and “enhanced-beta”).
There are additional competitors trailing behind such as Synthekine, Asher Bio, and Good Therapeutics (acquired by Roche). However, none of them have reported any clinical data to compare with other IL-2 clinical programs. Synthekine has a different view of the fundamental biology of IL-2, which is at odds with the whole field. Asher Bio and Good Therapeutics are both attempting convoluted solutions that carry additional uncertainty and biology risks, but investors should keep these companies on their radar as early trial data emerges in the future.
The many attempts at IL-2 optimization show just how enticing the concept of improved IL-2 is within the oncology field. Some of these failures were very expensive for Pharma – costing BMY and Sanofi multiple billions. While these disappointments are stark, they also reflect the immense value that is in play for getting this idea right. If the product profile of MDNA11 continues to look as good as it seems from early data, this would be incredibly lucrative for Medicenna and extremely attractive to multiple big pharma players. It would likely prompt a massive investment, either in the form of a partnership or outright buying of the asset/company. And this would not come cheap.
Medicenna’s Pipeline
Behind MDNA11, Medicenna is also developing an IL-4 Empowered Superkine, bizaxofusp (formerly MDNA55). Bizaxofusp has been studied in 130 patients among 5 clinical trials. Its lead indication is recurrent glioblastoma multiforme (rGBM), the most common and fatal form of brain cancer with terrible outcomes from extremely limited treatment options. Bizaxofusp is well-suited to target GBM since it consists of an engineered IL-4 cytokine molecule with a catalytic domain from Pseudomonas Exotoxin A attached as a lethal payload for attacking the brain tumor, a unique approach of achieving ADC like efficacy without the associated liabilities. Research shows that IL-4 receptor is overexpressed in brain tumor but not expressed in healthy brain tissue, creating an opportunity for targeted treatment that can spare the brain. Bizaxofusp is delivered via Convection Enhanced Delivery (CED) to bypass the blood brain barrier to accumulate drug at the IL-4 receptor in the brain tumor and avoid systemic toxicity risk. Bizaxofusp has completed a Phase 2b trial in GBM, and Medicenna recently reported updated results at the SNO conference in comparison to an external control arm (ECA). When compared to a well matched ECA, median overall survival was doubled by a single treatment with bizaxofusp. While Medicenna has been looking for a partner to further develop this program for over 2 years, and pharma has steered clear of the challenging GBM space, the FDA recently endorsed the use of an ECA as hybrid control in the bizaxofusp Phase 3 trial design in rGBM. This regulatory flexibility may prompt renewed investment in the GBM field. Furthermore, recent interest with Servier’s brain tumor program (vorasidenib) as well as Day One’s pediatric low grade brain cancer therapy (tovorafenib) seems to have generated further interest in the brain cancer field and therefore bizaxofusp may finally be ripe for a partnership and advancement into a Phase 3 clinical trial.
Medicenna also has an early-stage BiSKITs™ program (Bifunctional SuperKine ImmunoTherapies) under development to enhance the ability of Superkines to treat immunologically “cold” tumors. Bispecifics are currently enjoying a renaissance in the immuno-oncology space, and this technology platform could prove very valuable down the line.
Investors should own Medicenna (MDNAF) heading into Multiple Catalysts
While Medicenna continues to trade on the main Toronto Stock Exchange (TSX), the company decided to delist from NASDAQ in the US instead of using the well-known “reverse-split” to regain compliance. After delisting from NASDAQ, Medicenna now trades on the OTCQB under the ticker $MDNAF. This delisting was strategic as it reduced spending and extended the company’s cash runway from Q3 2024 to Q2 2025. This extended cash runway will ensure that Medicenna is fully capitalized through multiple catalysts and inflection points for further value creation. With additional MDNA11 data updates and potential for bizaxofusp partnership, the company will then be in a much more favorable financial position to raise money either via offering or partnership/buyout for further development. MDNA11 is highly discounted by the market as MDNAF trades around the level of its current cash reserves without even adding the value of its Phase 3-ready bizaxofusp or its Phase 2 drug program MDNA11. We believe this represents a market dislocation, since MDNA11 has the potential to become the best-in-class IL-2 approach, which is a highly sought after concept. Based on all the above, we think it is the best time to start building a position in $MDNAF shares and adding with any positive trial updates along the way in 2024.
Technical analysis
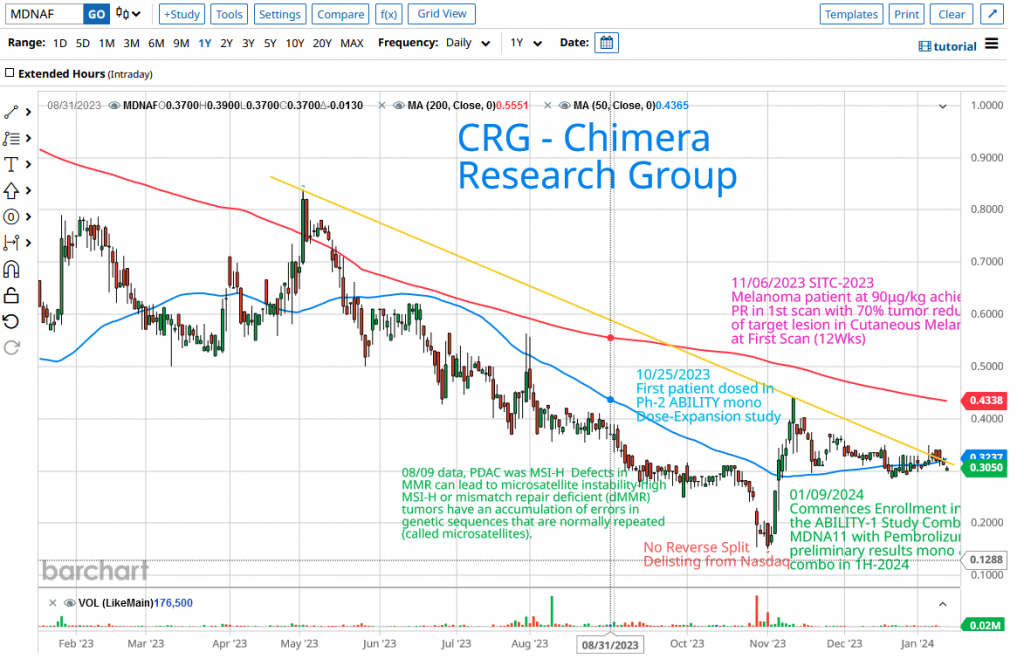
In a challenging year for the biotech sector marked by the XBI consistently printing a new low, the broader market surged, reflected in indices such as SPY and QQQ repeatedly hitting new 52-week highs. External factors like a high-interest-rate environment posed significant hurdles for microcap biotech firms seeking capital or approaching major pharmaceutical companies for partnership deals. Despite Medicenna presenting favorable data from its dose escalation trial, the company faced downward pressure, trading in a downtrend. Once investors realized the abandonment of plans for a reverse split, the stock swiftly rebounded from its 52-week low of $0.151 to reach $0.441, within just six trading days. Subsequently, it entered a period of sideways-trading, indicating consolidation in the $0.30-$0.35 range and holding above the 50-day moving average (represented by the blue line on the chart). The current price is nearing a critical point to challenge the downtrend line (yellow line). A breakout above the downtrend line would signal a new upward trend towards the 200-day moving average (the red line) around $0.44, followed by the 52-week high of $0.8188.
The Trade
Engaging in a speculative trade involves inherent high-risk dynamics but also offers the potential for significant rewards. The outlook for Medicenna hinges on its ability to yield enhanced tumor activity and positive responses (PRs or CRs) in the dose expansion cohort, as well as in the subsequent combination study. If Medicenna replicates or surpasses the favorable outcomes observed in the dose escalation cohorts while maintaining safety, there is a strong likelihood of a substantial upward movement in the share price.
Currently, MDNAF is trading under-the-radar, with an extremely low valuation, presenting an undervalued opportunity. The current market capitalization stands at approximately $29 million. I anticipate Medicenna to emerge as a potential “multi-bagger” trade, reaching at least $1.50 to $2.00 per share if the phase-2 dose expansion results prove positive (data in H1-2024). Further, any hint of a positive outcome from the combo trial with Merck’s drug Keytruda in the first half of 2024 (interim result) follwed by full results in the second half of 2024 could propel the share price to $5 or more. This might trigger speculation within the biotech community about Merck (or any other large pharma with a PD-1 inhibitor) considering a takeover of Medicenna.
With shares trading within the $0.30 to $0.35 range, now would be the best time to start building a position in $MDNAF (or in Canada $MDNA.TO around CA$0.40 to CA$050). Further additions to the position can be made following a breakout above the downtrend line on large volume. The completion of the full position can be progressively attained as time goes on, especially in response to positive trial updates anticipated throughout the year.
Risk Consideration
Like any other biotech venture, there is risk associated with clinical trials, and Medicanna shares a similar risk profile. If the MDNA11 study fails to demonstrate an increased number of positive responses (PRs), there may be a need for additional dilutive funds raising at a lower valuation to support the development of BiSKITs. Alternatively, reliance on a partnership for MDNA55 to progress into Phase 3 study could become crucial. Therefore, exercising caution and conducting thorough due diligence are imperative when considering investments of this nature.
Disclosure: Author is Long MDNA.TO and MDNAF













